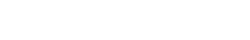ISR Platform®
A unique research tool for injection site reaction studies
Human response to your vaccine and drug candidates
First-in-human data without harming humans or animals

Local adverse events are too often discovered upon injection during clinical trials, causing unexpected pain to volunteers and resulting in lower patient compliance. Genoskin has designed a unique platform to study inflammatory side-effects to injections prior to clinical trials. Our ISR Platform® helps you assess and predict inflammatory response to subcutaneous, transdermal and intradermal vaccines, drugs and medical devices. A quick overview of the platform’s features and benefits:
Cutting-edge
Unbiased automated bioinformatics-based analysis of immunotoxicity and cytotoxicity.
Characterization
Deep knowledge of drug-induced pseudo-allergic reactions to better prevent and mitigate them.
Injections
Local biological response to self-injected drugs from live, immunocompetent human skin.
Adverse events
Predict the number one complaint from patients upon injection of biologics, prior to clinical trials.
Study human immunotoxicity and cytotoxicity in response to your drug
Custom experiments to help you move forward
Genoskin’s ISR platform® helps biotech and pharmaceutical companies understand and mitigate injection site reactions and determine the right formulation for their drug candidate, biosimilar or marketed drug. Depending on your needs, our expert immunologists design custom experiments to provide reliable answers and help you move forward safely.
Immunotoxicity
Local immune burst & inflammatory event associated with skin-resident immune cell or mast cell activation is mostly caused upon injection of biologic drugs.
Cytotoxicity
Local toxicity causing cell death and necrosis is mostly caused upon injection of small molecule drugs.
First-in-human data on immunotoxicity
Unbiased Multiplex Cytokine Assays



In order to help you predict active biological processes at the site of injection, we study the immunotoxicity of subcutaneous or intradermal drugs, vaccines and medical devices directly in our live and immunocompetent HypoSkin® models. This unique approach provides the missing link between preclinical and clinical trials. It helps you obtain live immune response to different formulations from a cohort of healthy skin models to better understand immune response.
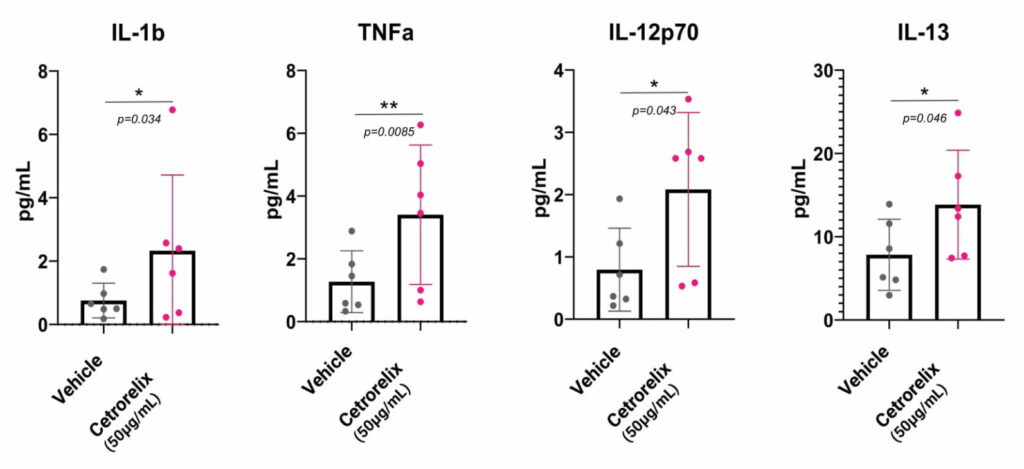

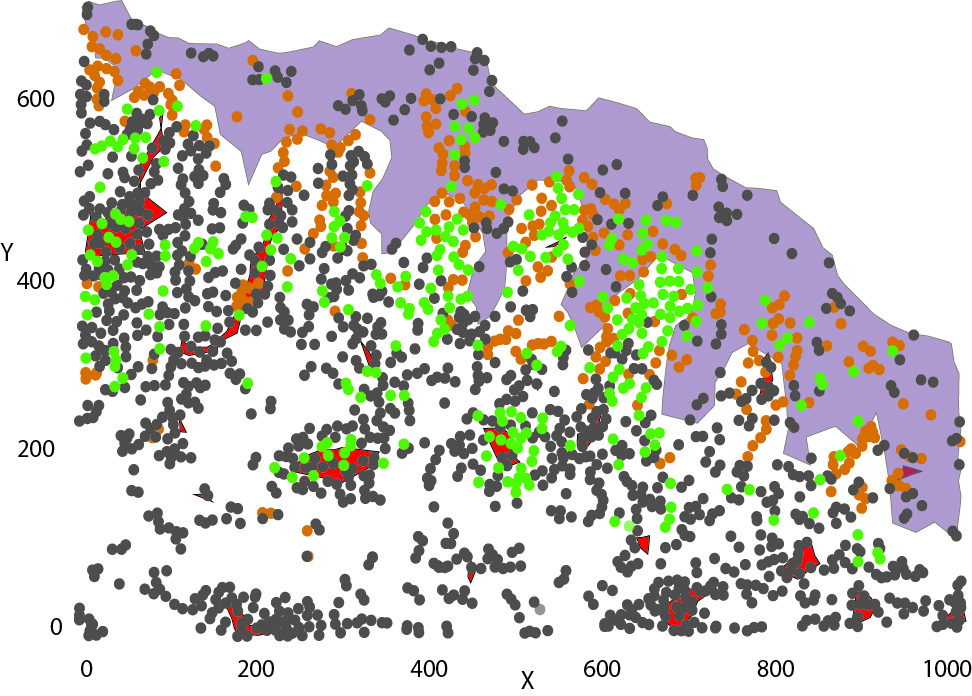
To collect all the relevant data, we simultaneously measure the proteins released in the HypoSkin® culture medium and matrix. Cytokines are dosed using the MesoScale technology. The data are then clustered using our proprietary AUDaCY (AUtomated Data Analysis of CYtokines) application, to profile inflammation at the proteomic level, highlight the mechanisms involved and associate real-world evidence to better predict clinical outcomes.
First-in-human data on cytotoxicity
Predict local toxicity for self-injected drugs
Genoskin’s ISR platform® helps you de-risk clinical trials via cytotoxicity studies on living human skin from a cohort of healthy donors. Genoskin’s ISR platform® helps biotech and pharmaceutical companies understand and mitigate injection site reactions and determine the right dosage for their drug candidate, biosimilar or marketed drug. When it comes to research on immunotoxicity, the platform generates first-in-human data on loss of cell viability, signs of cell death and cytokine release.
Identify loss of cell viability
To provide reliable data on loss of cell viability in human skin, our skin biology experts perform histological analyses to quantify pyknosis, epidermal detachment, papillary dermis alteration or breaks in adipocyte membranes.
Detect signs of cell death
Our experts perform a range of immunofluorescence assays, such as TUNEL assays, to detect DNA breaks. We also conduct spectrometry analyses of LDH release to measure cell death.
Explore cytokine release
To generate an injected drug’s proteomic signature and better characterise off-target events, we combine multiplex cytokine assays with Audacy, our proprietary software that allows automated clustering of cytokines for unbiased analysis.
Interested in seeing how others use our ISR® Platform?
Feel free to have a look at this case study to see how one of our customers mitigated injection site reactions using the ISR Platform®.
Take your research one step further
Discover what triggered the adverse event
Genoskin associates cutting-edge technology with proprietary AI-based analytical applications to help you understand the mechanism of action behind the adverse effect we observed in situ or following a clinical trial. We generate the unique biological signature left behind by your biotherapeutic agent to select the drug candidate or formulation that is least likely to trigger injection site reactions, combining our MANTIS® spatial biology platform and RNA sequencing.
Frequently asked questions
What are injection site reactions?
Corbière, A, Loste, A, Gaudenzio, N.- Exp Dermatol. 2021; 30: 193– 200.
Why study injection site reactions?
With the rapid growth of the biologic drugs class over the past two decades, a massive increase in unwanted immunogenic response has been reported. Too often, local adverse effects are only discovered at the clinical trial stage or when a drug is already on the market. As a consequence, they may delay the drug development process and increase associated costs or negatively affect patient compliance when discovered after the drug is put on the market.
Can I use multiple donors in one study?
How many donors and replicates does Genoskin recommend for statistically relevant results?
Does Genoskin offer diversity in donor selection?
What types of parallel testing does Genoskin offer?
What advantages does Genoskin ex vivo platforms offer for conducting toxicity studies?
- Human ex vivo skin platform: Genoskin’s non-clinical human skin platforms specialize in using donated skin, allowing for more relevant and translatable study results.
- Immunocompetent platform: Our technology acts as a proxy for studying the immune system, offering an immunocompetent platform that enhances the depth and relevance of your studies.
- Multiple donors per study: The ability to conduct studies on multiple donors ensures that the data generated is diverse and representative of either the general population or your target audience.
- Multiple conditions for one donor: Genoskin allows for the study of multiple conditions within a single donor, providing a more comprehensive understanding of local immune reaction to your compound.