Standardized ex vivo skin models for cosmetic testing
Cosmetic testing is playing an increasingly important role in today’s cosmetic industry. The first cosmetic tests took place in 1933, after an eyelash darkening treatment blinded several women. In 1938, the U. S. Food and Drug Administration passed the Federal Food, Drug & Cosmetic (FD&C) Act to define stricter regulations for cosmetic products (1). While the FD&C did not specifically require the use of animals in testing cosmetics for safety, the cosmetic industry increasingly resorted to animal experiments, which was a common scientific testing practice at the time. However, today, animal testing is leading to growing public outrage over the fate of laboratory animals and cosmetic manufacturers resort to new techniques to validate the safety and efficacy of their products.
Animal testing, an increasingly questioned practice
Even though animal testing has been used since ancient times to develop new medical and pharmaceutical products and techniques, ethical and scientific considerations on animal testing (2) as well as the growing public concern for the fate of laboratory animals has given the practice a serious blow.
As a consequence, lawmakers have gradually reduced the use of animals in cosmetic testing procedures. In 1998, the United Kingdom was the first to completely ban the use of animals for cosmetic testing purposes. The European Union followed by introducing bans on animal testing of cosmetic products and ingredients in September 2004 and March 2009, respectively. In March 2013, the full ban came into effect (3). Other countries are quickly following suit, forcing the cosmetic industry to come up with new alternatives to test their products (figure 1). Public opposition to animal testing is also growing very fast in many countries including in the US.
Moreover, using animals to test products that are destined for human skin is not the most adequate solution. Ethical concerns aside, animal testing is time-consuming, expensive and often ineffective (4) as animal skin is anatomically different from human skin. As an example, the skin tissue of rodents is much thinner than human skin, which has lead past studies to overestimate product absorption and penetration in human skin (5).
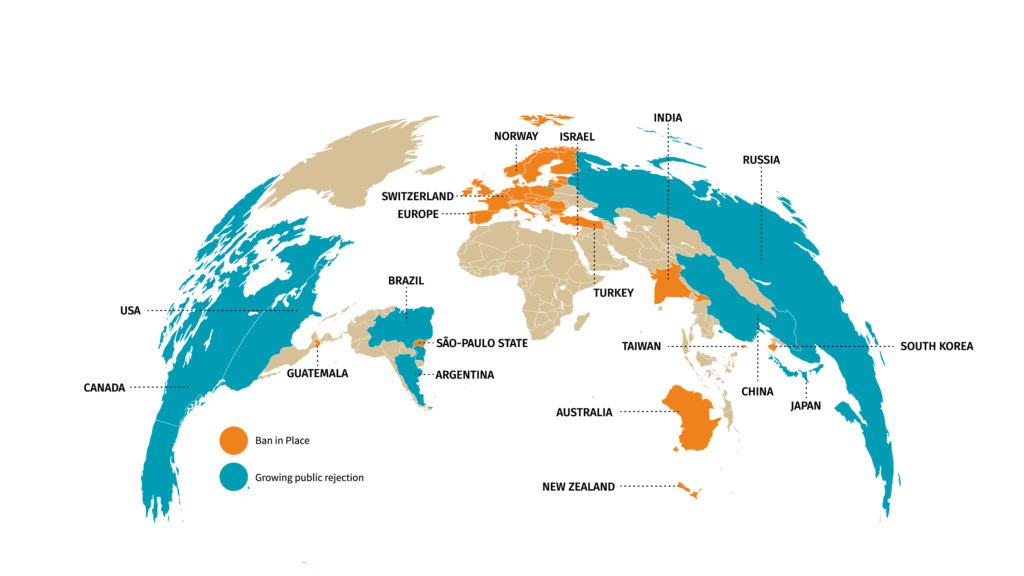
Figure 1: World map of restrictions on animal testing for cosmetics
The quest for alternative cosmetic testing tools
To help with the development and validation of alternative approaches, the EU released over €200m between 2007 and 2011. In 2011, a research initiative, called Safety Evaluation Ultimately Replacing Animal Testing (SEURAT) dedicated several tens of millions of Euros to the development of alternatives to animal testing for the prediction of repeated dose systemic toxicity.
In the US, the Johns Hopkins Centre for Alternatives to Animal Testing (CAAT) has been a leading force in promoting research into in vitro and other alternative techniques since it was founded in 1981. An American animal rights organization People for the Ethical Treatment of Animals (PETA) has also contributed with several millions of dollars toward the development of alternatives to animal use.
In vitro reconstructed human skin models for regulatory safety testing
These funds were mainly used to develop reconstructed in vitro models, which generally use a culture of primary human keratinocytes obtained from skin biopsies, but are sometimes also co-cultured with human fibroblasts on a collagen dermal substitute (6).
Different suppliers have developed a range of reconstructed human epidermis (RhE) models, which have been approved by the Organization for Economic Cooperation and Development (OECD) and validated by European Centre for the Validation of Alternative (ECVAM) for regulatory safety purposes (7). To measure skin corrosion, the following models have been validated (8): EpiDermTM (MatTek Corporation), EpiSkinTM (Episkin s.a.), SkinEthicTM RHE (Episkin s.a.) and epiCS® (CellSystems) models. To test skin irritation, cosmetic companies can use the following in vitro models (9): (modified) EpidermTM SIT, EpiSkinTM , SkinEthicTM RHE and LabCyte EPI-MODEL (Japan Tissue Engineering Co).
Skin bioprinting to industrialize the production of human skin models
More recently, skin bioprinting has emerged as a new tool to fully automate, standardize and reduce the production time of human skin models. Unlike conventional skin tissue-engineering approaches, this new manufacturing platform enables the simultaneous and highly specific deposition of multiple types of skin cells and biomaterials formulated into bio-inks. To date, while relatively simple human skin models with keratinocytes and fibroblasts have been successfully bioprinted, enormous challenges remain to completely replicate functional human skin (10).
The drawbacks of in vitro skin models
While in vitro reconstructed models have proven useful to assess the safety of cosmetic ingredients and products, they are not fully equivalent to in vivo human skin. The major drawback of these models is insufficient skin barrier function (11). Considerable efforts have been made to increase the complexity of in vitro models to mimic real, in vivo human skin.
However, to build more physiologically relevant human skin models, other skin components such as pigmentation, immunity, appendages, hypodermis, innervation and vascularization, need to be incorporated, which remains a serious challenge to this day (12). As a consequence, these models are less suitable for testing the efficacy of cosmetic products.
Ex vivo human skin samples for non-regulatory efficacy testing, a valid but imperfect alternative
In order to test cosmetics on real human skin without having to resort to in vivo models and without harming the patient, another practice is to use ex vivo human skin, which is generally obtained from cadavers or plastic surgery leftovers. Ex vivo human skin is considered the best surrogate for in vivo human skin (13): it holds normal skin barrier function and a mature stratum corneum.
A range of companies specialize in providing normal and diseased human skin tissue for testing purposes. Other companies known as CRO (Contract research organization) solely provide services for the in vitro and ex vivo testing of cosmetics on in-house ex vivo skin models.
The drawbacks of the current fresh and frozen human skin samples
Fresh human skin samples are mostly provided as raw skin samples and cellular viability rapidly decreases after tissue is removed from the patient’s body. Moreover, final users still need to prepare their samples and develop in-house tissue culture tools to keep the skin alive. In contrast, frozen skin is no longer viable (metabolically dead) and is no ready-to-use.
Genoskin’s NativeSkin® model: a fully functional ex vivo human skin model provided as a ready-to-use kit
The quest for an ideal ex vivo human skin model
Both the industry and academics are still on a quest to find the ideal human skin model, with the same barrier function, the same metabolic activity, immune response, functional skin appendages, dermal matrix with mechanical properties, a hypodermis layer and vascularization as in vivo human skin. Ideally, the model should be readily available, easy to use and provide reproducible study results.
Easy access to skin samples
The main objection to routinely using ex vivo human skin for cosmetic testing purposes is that human skin is difficult to obtain. However, due to the success of bariatric surgical procedures to treat obese patients, abdominoplasty procedures, also known as tummy tucks, are increasing worldwide, with over 700,000 procedures being performed in the world in 2016 and nearly 140,000 in the United States. Put all together, the total skin surface that could be obtained from these procedures annually is equivalent to about seven American football fields.
Most of this excess human skin tissue is disposed of by hospitals or clinics. Genoskin now retrieves this skin tissue, with the consent of the donor. It is transferred to the Genoskin team thanks to established tissue donation programmes between the donor site and Genoskin, in accordance with the Declaration of Helsinki and in full respect of all regulations.
The start-up then uses this skin to create patented, live ex vivo testing models. The NativeSkin® kit consists of full-thickness round human skin models prepared from the donated skin. The skin biopsies are embedded in a proprietary solid matrix, which maintains and nourishes the skin during shipment. It also contains a chemically defined animal-component free and xeno-free skin culture medium that keeps skin biopsies alive for up to 7 days (at 37°C with 5% CO2) (figure 2) (14).
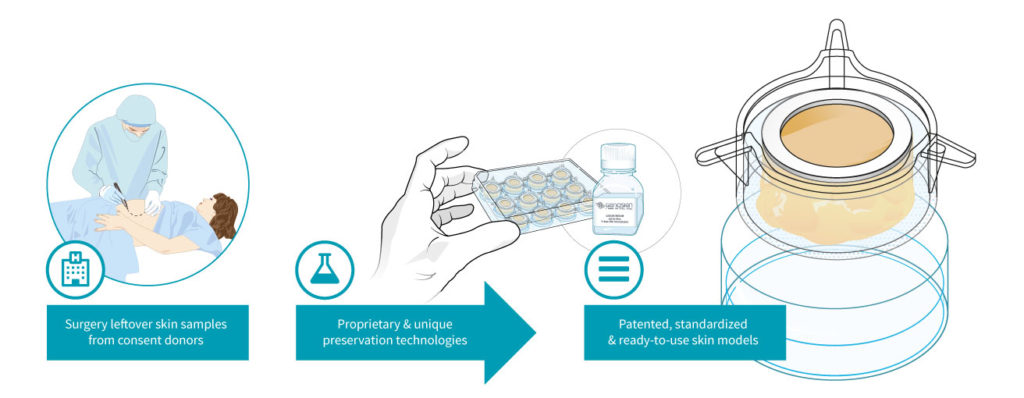
Figure 2: Genoskin’s unique NativeSkin® technology model
An ex vivo model with unique characteristics
Unlike other skin models, Genoskin markets unique, standardized and ready-to-use ex vivo human skin models.
- The skin models have the same structure as real human skin, with normal skin barrier function, a mature stratum corneum, a functional basal layer and they also include all the cell types (e.g. Langerhans cells) and skin appendages (i.e. sweat glands and hair follicle) of in vivo human skin;
- They provide live human skin response (15) that enables repeated testing for up to 7 days;
- The models are ready-to-use and easy to manipulate thanks to a clear and detailed user manual. Moreover, the models are produced in a standardized manner to guarantee reproducible results even when different donors are involved.
- Genoskin’s skin models are adapted to customer needs and come in different formats (i.e. biopsy diameters) to meet the needs for either topical or systemic administration. They include a flat silicone ring firmly fixed to the outer edge of the biopsy that prevents lateral diffusion of topically applied products and delimits a constant dosing area of topical application.
- Genoskin’s skin models are obtained with the donor’s informed consent and the approval of the Ethical committee/Institutional Review Board (IRB).
An enormous potential for cosmetic and pharmaceutical applications
Genoskin’s approach allows testing cosmetics on human skin without potential harm to the patient and without the need for animal testing. NativeSkin® ex vivo skin models are highly relevant to evaluate the skin permeation of topically applied cosmetic actives and to determine the efficacy and safety of cosmetic ingredients and products such as caffeine and parabens (16). Moreover, the model can be employed to assess the repeated topical applications of commercialized products for efficacy validation (15). This unique technology has proven useful to characterize the impact of indoor air pollution on skin enabling to detect changes in cell viability and proteasome activity (17). DNA damage in keratinocytes has also been reported in NativeSkin® skin models repeatedly exposed to UVB radiation and/or heat stress (18,19).
With a constant strive for innovation, Genoskin’s ex vivo skin model portfolio also offers the possibility to study adipose tissue reduction, hair growth mechanism and sebaceous gland function. Furthermore, this technology could be integrated to a ‘skin-on-a-chip’ system to have a fully vascularized and innervated ex human skin model (20).
This article first appeared under the title ‘Harm-free human testing’ in the February 2018 edition of Soap, Perfumery and Cosmetics magazine’s testing feature, vol 91 number 2.
For more information, don’t hesitate contact us.
References
(1) Willett J. The American beauty industry encyclopedia. Greenwood. 2010. (2) Ferdowsian HR, Beck N. Ethical and scientific considerations regarding animal testing and research. PLoS One. 2011, 6(9):e24059. (3) Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. (4) Hartung T. Opinion versus evidence for the need to move away from animal testing. ALTEX. 2017, 34(2):193-200. (5) Jung EC, Maibach HI. Animal models for percutaneous absorption. J Appl Toxicol. 2015, 35(1):1-10. (6) Almeida A, Sarmento B, Rodrigues F. Insights on in vitro models for safety and toxicity assessment of cosmetic ingredients. Int J Pharm. 2017, 519(2):178-185. (7) Zhang Z, Michniak-Kohn BB. Tissue engineered human skin equivalents. Pharmaceutics. 2012, 4(1):26-41. (8) OECD, July 2016. Test No. 431: In vitro skin corrosion: reconstructed human epidermis (RHE) test method. OECD Publishing. (9) OECD, July 2015. Test No. 431: In vitro skin irritation: reconstructed human epidermis (RHE) test method. OECD Publishing. (10) Ng WL, Wang S, Yeong WY, Nain MW. Skin bioprinting: impending reality or fantasy? Trends Biotechnol. 2016, 34(9):689-99. (11) Scientific Committee on Consumer Safety (SCCS). Basic criteria for the in vitro assessment of dermal absorption of cosmetic ingredients, SCCS/1358/10. 2010. (12) Abaci HE, Guo Z, Doucet Y, Jacków J, Christiano A.Next generation human skin constructs as advanced tools for drug development. Exp Biol Med (Maywood). 2017, 242(17):1657-1668. (13) Abd E, Yousef SA, Pastore MN, Telaprolu K, Mohammed YH, Namjoshi S, Grice JE, Roberts MS. Skin models for the testing of transdermal drugs. Clin Pharmacol. 2016, 8:163-176. (14) De Wever B, Kurdykowski S, and Descargues P. Human skin models for research applications in pharmacology and toxicology: introducing NativeSkin®, the “Missing Link” bridging cell culture and/or reconstructed skin models and human clinical testing. Appl In Vitro Toxicol. 2015, 1(1):26-32. (15) Norsgaard H, Kurdykowski S, Descargues P, Gonzalez T, Marstrand T, Dünstl G, Røpke M. Calcipotriol counteracts betamethasone-induced decrease in extracellular matrix components related to skin atrophy. Arch Dermatol Res. 2014, 306(8):719-29. (16) Duracher L, Visdal-Johnsen L, Mavon A, Kurdykowski S and Descargues P. A novel explant model for skin delivery assessment. Cosmet & Toil. 2015,130(2):30. (17) Dezest M, Le Bechec M, Chavatte L, Desauziers V, Chaput B, Grolleau JL, Descargues P, Nizard C, Schnebert S, Lacombe S, Bulteau AL. Oxidative damage and impairment of protein quality control systems in keratinocytes exposed to a volatile organic compounds cocktail. Sci Rep. 2017, 7(1):10707. (18) Calapre L, Gray ES, Kurdykowski S, David A, Hart P, Descargues P, Ziman M. Heat-mediated reduction of apoptosis in UVB-damaged keratinocytes in vitro and in human skin ex vivo. BMC Dermatol. 2016, 16(1):6. (19) Calapre L, Gray ES, Kurdykowski S, David A, Descargues P, Ziman M. SIRT1 activation mediates heat-induced survival of UVB damaged keratinocytes. BMC Dermatol. 2017, 17:8. (20) Wufuer M, Lee G, Hur W, Jeon B, Kim BJ, Choi TH, Lee S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci Rep. 2016, 6:37471.
Comments are closed.