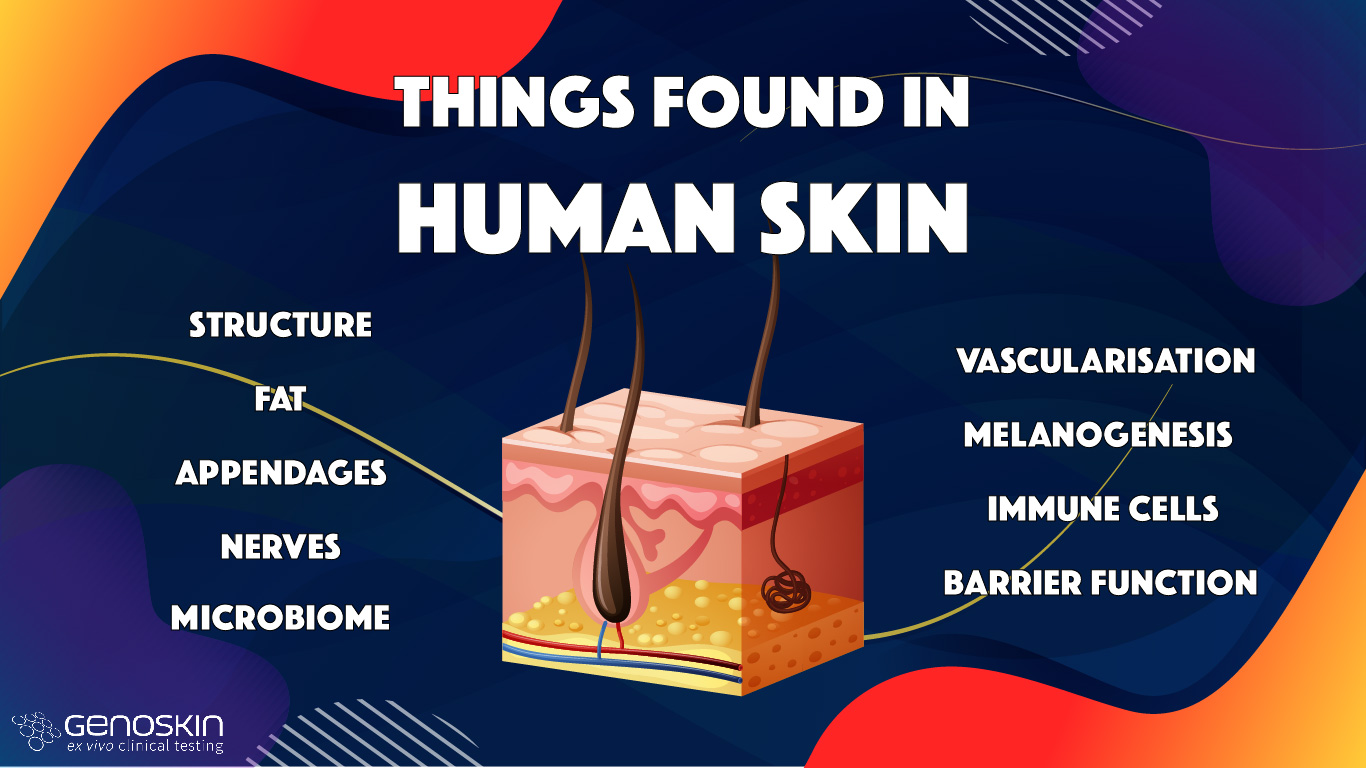
Top things found in human skin (vs in reconstructed human skin models)
Skin is our largest organ (2m2). Since the skin is a mandatory test organ for regulatory agencies, every single drug in development needs to be tested for potential skin toxicity. Today, efficacy and safety studies are performed either on animal skin, reconstructed skin, or ex vivo human skin. In a previous article, we discussed the top differences between animal and human skin. Let’s now uncover the differences between reconstructed and real native skin.
How are reconstructed human skin models used in research today?
Reconstructed human skin models were developed in the 1980s to substitute for animal experimentation (1). They aim to provide a more accurate and more ethical solution to researchers. Today, they are widely used in cosmetics research to assess the safety and efficacy of topical compounds. The market has grown fast as some governments banned the use of animals for cosmetics research and development. In the EU, reconstructed skin models are used for tests validated by OECD such as skin corrosion, skin irritation and dermal absorption tests.
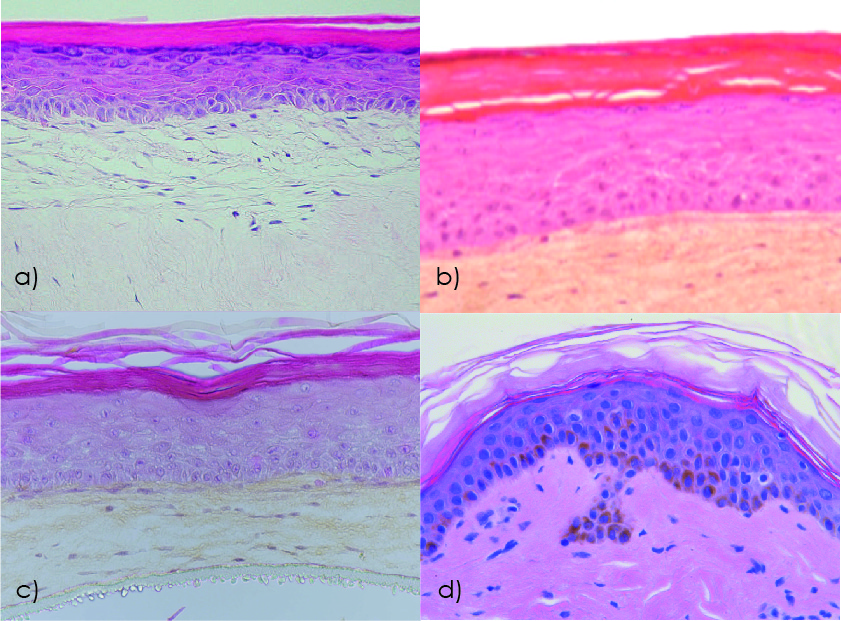 Figure 1. Human skin model histology studied by hematoxylin-eosin staining: a) Reconstructed human skin model EpiDermFT (MatTek); b) Reconstructed human skin model T-Skin (Episkin); c) Bioprinted human skin model Poieskin® (Poietis); d) Ex vivo human skin explant NativeSkin® (Genoskin). Modified from (2) and (3).
Figure 1. Human skin model histology studied by hematoxylin-eosin staining: a) Reconstructed human skin model EpiDermFT (MatTek); b) Reconstructed human skin model T-Skin (Episkin); c) Bioprinted human skin model Poieskin® (Poietis); d) Ex vivo human skin explant NativeSkin® (Genoskin). Modified from (2) and (3).
Over the years, reconstructed models have become more complex (Figure 1). Now, a new wave is coming with the development of 3D-bioprinting (4). How relevant are these models to real human skin? And can they be used for dermatology and drug development?
What are the top 10 differences between reconstructed and real human skin?
Skin basic structure
Human skin is a complex organ made up of 3 layers (epidermis, dermis and hypodermis), cell layers and cell types whose primary function is to act as a barrier preventing dehydration and keep most chemicals, toxins, bacteria and viruses out of the body.
Standard full-thickness reconstructed human skin models consist of a dermal equivalent overlayed by a stratified epidermal equivalent. To form the dermis, primary fibroblasts are seeded in a collagen matrix. Then, primary keratinocytes are cultured on the living dermal equivalent. Keratinocytes differentiate into 8-12 layers and form a stratum corneum at the tissue/air interface.
The main structural differences between these models and native human skin are the absence in reconstructed models (Figure 2) of a normal dermis with papillary and reticular layers, extracellular matrix made of hyaluronic acid, collagen I and III and elastins, as well as rete ridges at the dermal-epidermal junction (3).
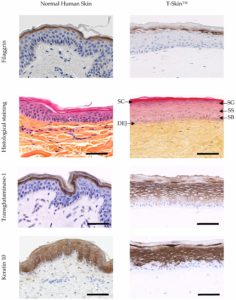
Figure 2. Characterization of the epidermis from normal human skin and reconstructed human skin (T-Skin™) by histology and immunohistochemistry (anti- keratin 10, Transglutaminase-1, Filaggrin) analysis on paraffin sections. Localization on histological stained images of the stratum corneum (SC), stratum granulosum (SG), stratum spinosum (SS), stratum basal (SB) layers of the epidermis and the dermo-epidermal junction (DEJ). Scale bar represents 100 μm. Modified from (3).
Presence of subcutaneous tissue
The hypodermis is the subcutaneous layer of the skin, located below the dermis. It is an endocrine organ that’s important for glucose homeostasis and lipid metabolism.
The hypodermis is mostly composed of fibrocytes and adipocytes and is also rich in proteoglycans and glycosaminoglycans. Its most known functions are to store energy in the form of fatty acids and to act as an insulating layer.
However, the adipose tissue also plays a role in the skin immune system producing mediators such as growth factors, adipokines, cytokines and chemokines that are released in the circulation (5).
Finally, subcutaneous injection has proven to be an efficient administration route for drugs. Indeed, the subcutaneous tissue can store the injected compound and gradually release it into the body.
Today, there are no commercially available reconstructed models that present subcutaneous tissue. Researchers have been working on different approaches to reconstruct 3D adipose tissue, including bioprinting but no extensive data is available (6).
Skin appendages
- Sweat glands
In human skin, there are three types of sweat glands: eccrine, apocrine, and apoeccrine. Eccrine glands are the most numerous and distributed across the whole body. It is responsible for the highest volume of sweat excretion. Apocrine and apoeccrine glands are only located in specific axillary regions. The main role of sweat glands is human thermoregulation.
- Sebaceous glands
In the human body, sebaceous glands are associated with hair follicles. Sebaceous glands are holocrine glands that secrete sebum. Their importance is still uncertain but sebum is thought to have antibacterial and antifungal properties.
- Hair follicles
The hair follicle plays a critical role in thermoregulation, dispersion of sweat and sebum, sensory and tactile functions, skin regeneration, and repigmentation.
To date, the inclusion of hair follicles, sweat or sebaceous glands in reconstructed or bioprinted skin models is still very limited and requires strong further development of technologies (7). Consequently, the absence of skin appendages in reconstructed or bioprinted skin models also means they are not suitable for testing hair care products or products targeting sebum production.
Peripheral nerves
The skin is a very sensitive organ densely innervated by a meshwork of peripheral nerves defined by the presence of specific receptors and involved in the perception of tactile mechanical stimuli and of noxious stimuli (e.g. pain, high temperature or irritant).
Free peripheral nerve endings can be found in the dermis (e.g. surround hair follicles) as well as deep in the epidermis. For example, motor nerves are sympathetic nerves located in the dermis that secrete neurotransmitters, neuromodulators, and neuropeptides to regulate sweat gland secretion, vasoconstriction, and temperature balance adjustment. Nociceptive sensory neurons (nociceptors) project both in the dermis and epidermis. Like resident immune cells, nociceptors represent a unique type of “skin‐resident cell” capable of responding to harmful stimuli from the microenvironment and regulating inflammatory response. Recently, skin-projecting nociceptors have been shown to interact with skin-resident immune cells (e.g. DC and mast cells) to regulate immune defenses against invading pathogens but also inflammation, pain and itch (7).
Obviously, only cut off nerve endings remain in skin explants because of the surgery procedure. Nevertheless, several researchers has demonstrated the possibility to innervate reconstructed human skin models using iPS (8), bringing hope to better understand the interaction of innervation and skin homeostasis.
Blood circulation
The skin is a highly irrigated organ. Blood and lymphatic vessels populate the dermis and the hypodermis. Interestingly, no vascular system is found in the epidermis. The skin vasculature system is a very complex network that is still very difficult to reproduce in vitro (9).
Many studies have reported the alignment of endothelial cells to vessel-like structures when these cells are added into the dermal part of a reconstructed skin model culture in vitro (10, 11, 12). Nevertheless, no functional perfusable vasculature is formed in vitro in these skin models.
Alternatives to these approaches include microfluidic-based skin-on-chip models that can theoretically reproduce physiological conditions (13) or the use a biological vascularized scaffold (BioVaSc) produced from a decellularized segment of porcine jejunum combined and combine to a bioreactor system (14).
Melanocytes and melanogenesis
In native human skin, melanocytes occur naturally and their density in the skin varies with the body site from around 900 melanocytes per square mm on the back to around 1500 melanocytes per square mm in the genital region (15). Variation of melanocyte density is remarkably small between individuals of different backgrounds (16,17). Their role is to produce the melanin that protects skin against the damaging effect of ultraviolet radiation.
Basic reconstructed skin models don’t present melanocytes. However, in some more complex models, melanocytes can be added. They are usually involved in pigmentation studies. More pigmentation in reconstructed tissues is only achieved by adding additional melanocytes, which is totally not physiological. Indeed, darker complexions in normal tissue are the result of higher melanin content, and not a greater amount of melanocytes.
The presence of functional skin resident immune cells
The skin immune system lies mostly in the epidermis and dermis, while some immune cells can also be found in the hypodermis (e.g. macrophages). The epidermis acts as the first barrier of defense against external hazards. It is populated with keratinocytes, Langerhans cells (LC) and ɑβ T cells. In the dermis, macrophages, various dermal dendritic cells (DC), mast cells and lymphocytes such as additional ɑβ T cells, natural killer cells as well as B cells can compose the immune system.
Commercially available reconstructed human skin models are known to lack immune cells. However, some in-house models started to integrate LC-like and/or T cells (Figure 3). One of the first immune reconstructed human epidermis was developed in 2005 and integrated CD34+ cord blood progenitor-derived LC. The model was used to study skin reaction to topically applied allergens. This RhE-LC-like model showed some donor variation with the majority of the donors responding to the allergens. The main limitations were the absence of the dermis, which plays an important role in skin sensitization, the logistics to culture primary keratinocytes and cord blood-derived LC-like together and the fact that in vitro-derived keratinocytes and LC-like might not recapitulate full biological features of in vivo native cells.
Finally, in addition to defending the host against damaging or potentially damaging environmental cues, the immune system might have additional important functions in the regulation of tissue homeostasis and physiological processes that will be absent in reconstructed human skin models.
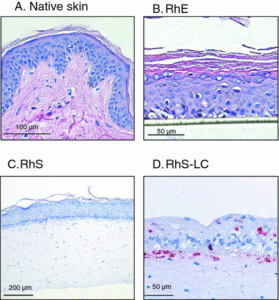
Figure 3. Histology (hematoxylin–eosin staining) of (a) native skin (biopsy), (b) reconstructed human epidermis (RhE) on a polycarbonate filter, (c) reconstructed epidermis on fibroblast-populated dermis (RhS), and (d) An HLA-DR immunohistochemical staining of MUTZ-LC integrated in reconstructed epidermis on fibroblast-populated collagen hydrogel (RhS-LC) (stratum corneum was lost during immunohistochemical staining procedure). Modified from (18).
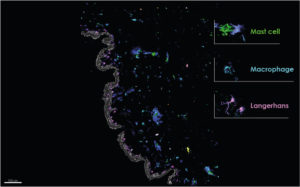
NativeSkin® multiplex imaging. Genoskin.
Skin barrier function
Human skin is a shield regulating the penetration of water, nutrients, ions and environmental stimuli. The skin protects the diffusion of molecules, chemical exposure, ultraviolet radiation and penetration of pathogens. It also protects against dehydration. The skin barrier function maintains tissue homeostasis.
We can distinguish four component of the skin barrier:
- Physical: it’s the first barrier composed by the stratum corneum and tight junctions. The stratum-corneum is considered an outside-in barrier as it prevents penetration of external elements into the skin. Tight junctions represent an inside-out barrier preventing body dehydration. In vitro 3D skin models have been extensively used to study the physical barrier. However, despite years of optimization, reconstructed and bio-printed models still show differences in the stratum corneum from native skin. Indeed, they present differences in lipid composition and organization leading to higher permeability (19).
- Chemical: the release of defense molecules composes the chemical barrier. Those defense molecules are mainly antimicrobial peptides as well as ROS. This barrier has been less studied in 3D skin models and is a field of optimization.
- Immunological barrier: This is the secondary barrier in case of a defect in the physical barrier. Langerhans cells, keratinocytes and other skin resident immune cells, including dermal dendritic cells, mast cells or T cells, are the main actors of this barrier. The interplay between keratinocytes and skin resident immune cells is vital for tissue homeostasis and inflammatory processes. This barrier is also less studied in 3D skin models as there has been no reconstructed model presenting all immune cells. Immunological barriers are also difficult to study in a static in vitro system.
- Microbial barrier: this is the skin microbiome. As of today, a large number of studies are conducted on skin microbiomes and on seeding whole patient-derived microbiomes on reconstructed models. However, the complex host-microbe and microbe-microbe interactions on the surface of human skin are still poorly understood.
Microbiome
An intact microbiota is essential to tissue homeostasis as it helps support the skin barrier. 3D reconstructed skin models are per se sterile and don’t contain any microbe. Researchers are able to selectively colonize those models by specific microorganisms. However, single bacteria strains don’t reflect the complex human skin microbiome (20).
Recently, reconstructed skin models designed to host microflora became available on the market. These models are either colonized with specific strains or with commensal skin microbiota. This is a step forward to study the interactions between host and microbe but there is still a strong need for experimental strategies for the colonization of 3D skin models with complete microbiota communities directly isolated from humans to mimic in vivo skin-microbiota interplay.
The human skin microbiota is acquired through an individual’s lifetime and some of its components are yet to be described. This makes it difficult for scientists to reproduce real human microbiota on reconstructed skin models.
Conclusions
In conclusion, reconstructed human skin models are an upgrade from animal skin in terms of relevancy and ethics. However, they still lack skin appendages, immune system, and hypodermis. These models do not yet support studying the efficacy and safety of non-topical compounds. This represents the need for most drugs on the market today.
Human skin samples can be kept alive outside of the human body for weeks. Therefore, ex vivo skin models are robust enough to support toxicity and efficacy testing of pharmaceuticals, chemicals, and cosmetics. They will also yield more relevant and comprehensive data. Real human skin models will always yield the best, most relevant and most reliable results.
References
- Nakamura, M, Haarmann‐Stemmann, T, Krutmann, J, Morita, A. Alternative test models for skin ageing research. Exp Dermatol. 2018; 27: 495– 500.
- Sheasgreen J, Klausner M, Kandárová H, Ingalls D. The MatTek story – how the three Rs principles led to 3-D tissue success! Altern Lab Anim. 2009 Dec;37(6):611-22.
- Bataillon M, Lelièvre D, Chapuis A, et al. Characterization of a New Reconstructed Full Thickness Skin Model, T-Skin™, and its Application for Investigations of Anti-Aging Compounds. Int J Mol Sci. 2019;20(9):2240. Published 2019 May 7.
- Derr K, Zou J, Luo K, Song MJ, Sittampalam GS, Zhou C, Michael S, Ferrer M, Derr P. Fully Three-Dimensional Bioprinted Skin Equivalent Constructs with Validated Morphology and Barrier Function. Tissue Eng Part C Methods. 2019 Jun;25(6):334-343.
- Nguyen AV, Soulika AM. The Dynamics of the Skin’s Immune System. Int J Mol Sci. 2019;20(8):1811. Published 2019 Apr 12.
- Heraud, Sandrine & Albouy, Marion & Marquette, Christophe & Thepot, Amelie & dos Santos, Morgan. (2018). Adipose Tissue-engineering: bioprinting of a human functional 3D-hypodermis.
- Randall MJ, Jüngel A, Rimann M, Wuertz-Kozak K. Advances in the Biofabrication of 3D Skin in vitro: Healthy and Pathological Models. Front Bioeng Biotechnol. 2018 Oct 31;6:154.
- Muller Q, Beaudet MJ, De Serres-Bérard T, Bellenfant S, Flacher V, Berthod F. Development of an innervated tissue-engineered skin with human sensory neurons and Schwann cells differentiated from iPS cells. Acta Biomater. 2018 Dec;82:93-101.
- Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011 Apr 30;63(4-5):300-11.
- Lei XH, Ning LN, Cao YJ, Liu S, Zhang SB, Qiu ZF, Hu HM, Zhang HS, Liu S, Duan EK. NASA-approved rotary bioreactor enhances proliferation of human epidermal stem cells and supports formation of 3D epidermis-like structure. PLoS One. 2011;6(11):e26603.
- Montano, I., Schiestl, C., Schneider, J. et al. (2010). Formation of human capillaries in vitro: The engineering of prevascular-ized matrices. Tissue Eng Part A 16, 269-282.
- Tremblay, P. L., Hudon, V., Berthod, F. et al. (2005). Inoscu-lation of tissue-engineered capillaries with the host’s vas-culature in a reconstructed skin transplanted on mice. Am J Transplant 5, 1002-1010.
- A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015;15:2688–99
- Groeber F, Engelhardt L, Lange J, Kurdyn S, Schmid FF, Rücker C, Mielke S, Walles H, Hansmann J. A first vascularized skin equivalent as an alternative to animal experimentation. ALTEX. 2016;33(4):415-422.
- Jorizzo JL, Bolognia JL, Rapini RP. Dermatology: 2-Volume Set: MOSBY. 2008. (ELSEVIER)
- Alaluf S, Atkins D, Barrett K, Blount M, Carter N, et al. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res. 2002;15:112–118.
- Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, et al. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124:1326–1332.
- Rodrigues Neves C, Gibbs S. Progress on Reconstructed Human Skin Models for Allergy Research and Identifying Contact Sensitizers. Curr Top Microbiol Immunol. 2018 Jun 23.
- Niehues, H, Bouwstra, JA, Ghalbzouri, AE, Brandner, JM, Zeeuwen, PLJM, van den Bogaard, EH. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Exp Dermatol. 2018; 27: 501‐ 511.
- Rademacher, F, Simanski, M, Gläser, R, Harder, J. Skin microbiota and human 3D skin models. Exp Dermatol. 2018; 27: 489– 494.
Comments are closed.